Success stories from projects
Two-dose Ebola vaccine is safe, induces robust immune response in adults with HIV
12/11/2021Data supports the IMI/Johnson and Johnson vaccine regimen as a preventative against Ebola in risk areas.
Quality matters: making sure research data is fit for its intended use
08/11/2021The EQIPD team developed a wiki-based quality system to help increase adherence to rigorous, evidence-based practices in preclinical research. Interview with Malcolm McLeod

Multiple important milestones advance Ebola vaccine regimen uptake
26/10/2021European marketing authorisation, WHO prequalification and an expert-backed recommendation mean the IMI-funded J&J vaccine is closer to widespread uptake to prevent future outbreaks.

Scientists discover a highly potent antibody against SARS-CoV-2
19/10/2021Researchers in Switzerland, working as part of the CARE consortium, have isolated a highly potent monoclonal antibody against SARS-CoV-2, one of the most powerful identified so far.

Surge vaccine production principles now written into EU law
13/10/2021ZAPI’s work can help dramatically speed up the development and approval of new shots for COVID strains, but also flu, malaria and other infectious diseases

Closing in on COVID: results from the first year of CARE
05/10/2021Multiple studies from the CARE project teach us more about how COVID19 wreaks havoc, and how it might be defeated.

Data suggest four categories of trajectories in people at risk of dementia
28/09/2021EPAD’s computational models, working on geographically-diverse, high-quality datasets, help identify subgroups in cognitive function evolution.
New non-profit organisation to help usher in EU’s networked and harmonised health data space
17/09/2021EHDEN generates evidence for diseases and treatments using a network of nearly 100 data partners and over 400 million electronic health records.

Clinical trials for infectious disease research, on demand
15/09/2021The COMBACTE projects are the foundation of ECRAID, a network to support the full range of clinical research into new antimicrobial agents
To quarantine or not to quarantine? Predictive models can guide doctors’ judgement
03/09/2021DRAGON’s simple website can help speed up the use of machine learning tools to improve COVID care.

Results roundup: 13 years of diabetes breakthroughs
01/09/2021A summary of results of IMI diabetes research illuminates the sheer breadth of achievements, and recommends a path forward for making the most of them.

Identification of patient subgroups could pave way for personalised Sjögren’s syndrome treatments
17/08/2021There is currently no treatment for primary Sjögren’s syndrome, an autoimmune disease. New findings from IMI’s PRECISESADS project could help to change that.

By linking up hospitals, the study of antibiotics in drug-resistant infections just got easier
02/07/2021COMBACTE’s networks are helping overcome a persistent bottleneck in testing drugs against AMR – the relatively small numbers of cases in individual hospitals
A one-stop-shop for disease-specific human stem cells allows scientists to focus on research
21/06/2021Before the establishment of EBiSC, sharing disease-specific stem cell lines for research and development was fraught with challenges.

Drug safety academic programme expands to North America
14/06/2021Eu2P have offered a growing suite of certified drug-safety education courses since 2009, with renewed interest in the discipline spurred by COVID19. They are now expanding beyond the EU.

The most objective measure of your sociabilty? It’s in your pocket
11/06/2021PRISM is in talks with the EMA on the use of data from smartphones and wearables as biomarkers of social functioning in neuropsychiatric disorders.

Diabetes treatments set to get personal, thanks to new IT tool
08/06/2021Building on ground-breaking results from IMI diabetes projects, scientists are now working on a software tool that would identify what subtype of diabetes a patient has and suggest which treatment would work best for them.

INNODIA diabetes findings prompt new research into other autoimmune diseases
01/06/2021Most research into autoimmune diseases focuses on the role of the immune system. But INNODIA research underscores the importance of studying the tissues under attack as well.
The real cost of RSV, a common but often fatal disease
17/05/2021RESCEU have put together the most comprehensive cache of evidence to date on the true burden of a disease that still claims millions of mostly infant victims every year

EHDEN health data academy welcomes 1 000th student
05/05/2021Over 1 000 people have signed up to the EHDEN Academy, which provides the global scientific community with free educational resources on real world data and real world evidence.

Digital biomarkers: an initiative to help researchers navigate new reality & roles
05/05/2021A joint vision from MOBILISE-D and IDEA-FAST to strengthen R&D in the area of remote monitoring

New institute to act as catalyst for wider use of real-world evidence in healthcare decision-making in Europe
29/04/2021The GetReal Institute will be a focal point for information and exchange on the use of RWE in the drug development process.

Placebo response is affected by the drug you *think* you might be taking – especially if it’s an opioid
21/04/2021EUROPAIN showed that cautious description of expected drug efficacy and side effects may reduce the placebo analgesic response
Big data study leads to identification of blood biomarkers for Alzheimer’s
21/04/2021EMIF’s datasets helped researchers identify plasma proteins that changed in the presence of traditional Alzheimer’s markers, opening new avenues of research
US regulator to expedite review of gut microbiome protector product from COMBACTE partner
16/04/2021The US FDA grants ‘fast track designation’ to Da Volterra’s DAV132 for the prevention of Clostridioides difficile infection
Neuropathic pain was classified in a way that stymied research. Not anymore
15/04/2021EUROPAIN’s evidence that neuropathic pain patients should be grouped by their pain perception and not the event that sparked it has convinced the European regulator to change its guidelines
Machine learning models outperform traditional selection methods for recruiting osteoarthritis trial patients
08/04/2021APPROACH showed that the models were better at predicting whose disease would progress, thereby making drug testing more effective
A quick, cheap and non-invasive way to measure osteoarthritis
26/03/2021APPROACH found that a commercially-available motion-measuring technology could be used to gauge the extent of OA as an alternative or add-on to existing diagnostic options

A recipe for the next disaster: a new, pan-virus methodology for ramping up vaccine production
24/03/2021ZAPI have put in place a new pan-virus methodology for ramping up the production of new vaccines in the next crisis
Alzheimer’s: visual assessment of [18F]flutemetamol PET can reveal not only presence but also extent and location of amyloid
17/03/2021AMYPAD researchers have shown that a ‘visual read’ of PET scans using radioactive diagnostic tracer flutemetamol can go beyond simple yes-or-no determination of the presence of Alzheimer’s hallmark protein.

Latest COVID-19 vaccine based on technology tested for safety & immunogenicity in IMI Ebola projects
11/03/2021Safety and immunogenicity data on the Johnson & Johnson vaccine technology was gathered during IMI EBOVAC clinical studies.

TRIC-TB starts clinical trial of anti-TB drug booster
10/03/2021If approved for use, BVL-GSK098 could allow doctors to reduce the efficacious dose of two potent drugs used to treat tuberculosis, along with the side effects they cause.

Focus on: rare diseases
03/03/2021Science has tended to neglect diseases that affect small numbers of people, but that is changing.

Regulators clear study of gut microbiome ‘protector’ in cancer patients
16/02/2021COMBACTE-NET partner Da Volterra can move forward with phase 3 trial of an innovative product used to protect the microbiome of patients from antibiotic-induced disruption

Focus on: coronavirus
29/01/2021In March 2020, IMI responded rapidly to the emerging global COVID-19 pandemic. 144 applications led to 8 projects, and the funding pot was increased from €45 million to €72 million. Read about the projects and communications activities surrounding the Call.

New report highlights socio-economic impacts of IMI projects
13/01/2021Are IMI projects delivering socio-economic benefits? Yes, but it often takes time for these to become apparent, concludes a new report on the outcomes of 44 IMI1 projects.
IMI supported antibiotic passes Phase I clinical trials
14/12/2020A study in healthy volunteers showed that the antibiotic EBL-1003 is safe and well tolerated. EBL-1003 shows promise as a treatment for complex drug-resistant infections.

New brochure: IMI – radical collaboration in action
08/12/2020A new brochure showcases success stories from IMI projects in infectious diseases, brain disorders, diabetes, cancer, and other areas.
Focus on: technology convergence
30/11/2020IMI can provide a transparent, neutral and, importantly, pre-competitive setting that can provide regulators with solid evidence that new technologies are reliable. The technology will move very quickly, so we should, too.
Patient education project goes global with Japan national platform
04/11/2020Former IMI patient education project EUPATI is going global with the launch of a national platform in Japan, and the translation into Japanese of its toolbox. The move highlights EUPATI’s role as a global pioneer of patient education.

Major IMI patient education project embarks on new chapter
21/10/2020IMI patient education project EUPATI has set up an independent, non-profit foundation to build on the project’s work. The creation of the EUPATI Foundation secures the project’s legacy and paves the way for the further development of patient education resources in Europe and beyond.

UPDATED: Meet the IMI projects already helping to fight COVID-19
16/10/2020A number of IMI’s projects are making valuable contributions to the global effort to tackle COVID-19. The contributions include knowledge, tools and expertise, and while some come from projects in the infectious disease field, projects working in other areas, such as data management and Alzheimer’s disease, are also stepping up to the plate.
Two IMI projects to work with EMA on COVID-19
30/07/2020The EHDEN and ConcePTION projects plus the ADVANCE/VAC4EU will help the European Medicines Agency (EMA) gather real world data on COVID-19 vaccines and treatments once they are approved and being used in day-to-day clinical practice.
Regulatory decision gives boost to development of potential new TB drug
16/07/2020A decision by US regulators is set to speed up reviews of a potential new TB drug being developed by IMI’s TRIC-TB project.
Previous IMI investments are proving prescient in the current COVID-19 crisis
10/07/2020Investment in AMR, vaccines and infectious disease preparedness research are proving extremely prescient in the current pandemic, as are the relationships and networks we’ve built around the globe.
IMI-supported Ebola vaccine regimen gets green light
01/07/2020The European Commission has officially granted market authorisation for an IMI-supported Ebola vaccine regimen, which represents a vital tool in the fight against the deadly disease.
Focus on: autoimmune diseases
29/06/2020During the month of June we took a look at some of our autoimmune disease research projects.
New tools to help identify potential targets in autoimmune diseases
15/06/2020Unexplored proteins could be targets for new drugs to treat autoimmune and other diseases, if only we knew more about them. ULTRA-DD has brought us a few steps closer.
Proposed ‘clustering’ of patients offers hope for better understanding of autoimmune diseases
08/06/2020PRECISESADS’ findings show that despite being diagnosed with an array of autoimmune diseases, patients can be grouped according to molecular patterns
Crucial step towards EC regulatory approval of IMI-funded J&J Ebola vaccine
29/05/2020Studies indicate that the vaccine regimen is well tolerated, with robust and durable immune responses to the Zaire ebolavirus strain.
More - and better - patient data, whatever the disease
28/05/2020The generic architecture of RADAR-Base forms a reusable, flexible IT platform that allows for the remote monitoring of different diseases and conditions using a large variety of technologies.

The story so FAIR
13/05/2020Making data FAIR could supercharge medicine development. FAIRplus is putting the principles into practice.
Socio-economic legacy: EHR4CR's data reuse revolution
30/04/2020Electronic health record (EHR) systems are widely used in Europe and worldwide. These rich data sources can be used to address bottle necks in clinical trial design and, crucially, patient recruitment. However the lack of interoperability between EHR systems has traditionally limited the ability to efficiently combine the data across large populations for research analysis.

Socio-economic legacy: the European Lead Factory
23/04/2020The European Lead Factory is a collaborative public-private partnership that delivers innovative drug discovery starting points.

Socio-economic legacy: GetReal's real-world resonance
16/04/2020IMI project GetReal and its follow-up, GetReal Initiative, show new methods of RWE collection and synthesis could be adopted earlier in pharmaceutical R&D and the healthcare decision making process.
INNODIA set to speed up clinical trials for type 1 diabetes drugs
07/04/2020The INNODIA project’s clinical trial master protocol means launching new clinical trials for drugs to cure type 1 diabetes will be faster and easier.
Focus on: socio-economic impact of IMI projects
02/04/2020In April, it's IMI's birthday, 12 years and 150 projects later, what kind of socio-economic impact has IMI-funded research had?

IMI networks are helping set up and run COVID-19 drug trials
30/03/2020IMI’s COMBACTE project is perfectly placed to support the DisCoVery studies of four potential COVID-19 therapies
New tools and trials combat the resurgence of whooping cough
30/03/2020EU-funded researchers hope a greater understanding of interactions between pertussis bacteria and the immune system, together with a toolkit for testing new vaccines, will help prevent whooping cough disease and deaths in babies worldwide.
CANCER-ID hunts for cancer clues in blood samples
20/02/2020Today, many cancer patients have to undergo biopsy surgery to provide doctors with the cell samples they need to diagnose the disease and monitor how well a treatment is working. IMI’s CANCER-ID project has paved the way for the use of ‘liquid biopsies’ drawn from a blood sample.
First batch of real-world data from prostate cancer studies added to big data platform
13/02/2020Partners in the PIONEER project have uploaded datasets from major studies, and are calling on others to do the same

PARADIGM: a ‘game changer’ for patient engagement in R&D
30/01/2020When and how should the health research and development community involve patients in their work? IMI’s PARADIGM project is trying to find out.

Identifying potential antibiotic leads is painfully slow, but these researchers identified 5 in only 6 years
28/01/2020ENABLE surpassed their goal of identifying 3 leads for new antibiotics. With more promising compounds in the pipeline, the project has been extended.

A school for patients? Yes, you can be an expert in that
21/01/2020Grassroots campaigners paved the way for the modern patient advocacy movement, which is becoming increasingly sophisticated

Web search keywords can help spot early Alzheimer’s cases
16/01/2020Researchers from MOPEAD tested different ways to engage patients, including reaching out to suspected early Alzheimer’s sufferers by flagging internet searches like “forgetfulness”

PET scans that show brain changes caused by Alzheimer’s give doctors confidence in diagnosis
20/12/2019Being able to visualise the pathology linked to the disease is a big benefit for diagnosis and patient management. Researchers from an IMI project that set out to get definitive data on the role of amyloid PET scans in Alzheimer’s disease diagnosis are hearing from physicians that being able to visualise the underlying pathology linked to the disease is a big benefit for diagnosis and patient management. The ability to...
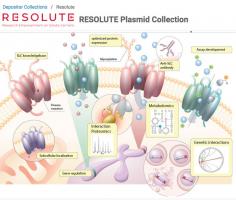
Cache of research materials on understudied proteins now available to scientific community
12/12/2019The RESOLUTE project have made the DNA reagents for 446 solute carriers freely available in the hope that the proteins might be used as targets in drug development
Alzheimer’s project EPAD releases first wave of data to research community
12/12/2019The data comes from EPAD’s study into the earliest stages of Alzheimer’s dementia – before symptoms appear.
Alzheimer's researchers use AI to get access to brains
10/12/2019The Aetionomy project used digital technology to study brains without needing samples from living people, allowing them to simulate processes associated with Alzheimer's.
Genetically engineered antibodies cut infection rates in a trial. Could they wean us off antibiotics?
04/12/2019Researchers’ hopes are raised after a trial using monoclonal antibodies to prevent pneumonia in ventilated intensive care patients gets good results
Can real world data replicate a clinical trial? EHDEN study suggests yes
26/11/2019IMI’s EHDEN project dramatically demonstrated the power of using clinical data in research by replicating, during a five-day ‘study-a-thon’, the results of a systematic review covering 20 years of research, and a multi-year clinical trial.
A very promising new antibiotic just started clinical trials
19/11/2019Researchers from ENABLE are hopeful about apramycin because it has passed the stage at which most potential antibiotics fail.

Why it’s so hard to make new antibiotics
15/11/2019There’s no lack of chemicals that can kill bacteria. One of the main reasons there have been so few new antibiotics developed in recent decades – apart from a poor return on investment – is that researchers have run out of ideas on how to outwit the protective mechanisms of Gram-negative bacteria.
Focus: Antimicrobial resistance
04/11/2019Antimicrobial resistance is becoming one of the defining problems of our time. As bacteria become resistant to the drugs that are supposed to kill them, scientists, policy makers and the pharma industry are looking at different ways to fix the problem before it’s too late. IMI has invested heavily in AMR research. To date, we have invested almost €800 million on 14 projects. In November, we will be taking a...
Trial of second Ebola vaccine to start in Democratic Republic of the Congo
31/10/2019The trial of the two-dose Ebola vaccine regimen aims to help tackle the current outbreak and strengthen future Ebola epidemic preparedness. An international consortium will soon start a large-scale clinical trial of an Ebola vaccine regimen in the Democratic Republic of the Congo (DRC), which is currently experiencing the second worst Ebola epidemic in history. IMI has contributed significantly to the development of the two-dose vaccine regimen, which is manufactured by the Janssen Pharmaceutical Companies of Johnson & Johnson.
Project to team up with African manufacturer to make diagnostic tests
28/10/2019This project is trying out a different kind of capacity building. Apart from hosting workshops and info sessions on the use of VHFMoDRAD's diagnostic tests, the project plans to identify and team up with a Senegalese producer that can make the tests themselves.
Data-driven personalised care for Alzheimer's patients
07/10/2019EU and industry-funded researchers are testing wearable devices, Internet of Things technologies and smartphone apps to transform the lives of people with Alzheimer's disease.
Focus on: Ebola
03/10/2019During the month of October we will be taking a look at some of the results of our Ebola-related research projects.
Two genes singled out as significant in the development of Alzheimer's
01/10/2019The PHAGO project has identified two genes that might have a significant impact on Alzheimers disease development and progression.
CHEM21 – a step towards a greener and more efficient pharmaceutical industry
11/09/2019The green manufacture of pharmaceuticals and production-related environmental concerns are high on the EU agenda, with the European Commission issuing the ‘European Union Strategic Approach to Pharmaceuticals in the Environment’ in March 2019. The Commission plans to encourage pharmaceutical companies to consider the environment more in the way they design and manufacture products. IMI’s CHEM21 project dealt with the sustainability of drug manufacturing processes, aiming to reduce the industry's carbon footprint and environmental footprint. The achievements of this highly successful project are transforming the pharmaceutical industry’s manufacturing paradigm and providing savings that can be reinvested in the development of new medicines.
Diabetes project leads to the world’s first validated human beta cell line
28/08/2019IMI’s IMIDIA project set out to study the function of a certain type of cells found in the pancreas which, when malfunctioning or weak, are thought to cause diabetes. We spoke to three of the researchers involved in the project about one of its biggest achievements: the identification and validation of a human beta cell line that behaves in the lab in the same way as the cells found in the human body.
EBiSC allows researchers to accelerate and expand their work
22/08/2019The European Bank for induced pluripotent Stem Cells (EBiSC) delivered a ground-breaking, sustainable repository of induced pluripotent stem cells (iPSCs), which now provides scientists across Europe with access to over 800 disease-relevant and quality controlled iPSC lines. In an interview with the IMI Programme Office, coordinators Andreas Ebneth, Scientific Director of Janssen Pharmaceutica NV, and Aidan Courtney, CEO of Roslin Cells Sciences discussed how the EBiSC infrastructure is facilitating and accelerating future discoveries and the development of new therapies.
Preparedness key to preventing Ebola outbreaks, says top African scientist Nicolas Meda
19/08/2019In 2014, an Ebola outbreak kicked off in the west African nation of Guinea and quickly spread to Sierra Leone and Liberia, putting other countries in the region on high alert. Epidemiologist and public health expert Professor Nicolas Meda of Burkina Faso played a key role in his country’s response to the epidemic, and participated in the IMI Ebola vaccine project EBOVAC2. In an interview with the IMI Programme Office, he looks back on the experience and sets out the lessons learnt and actions taken since then to prevent future outbreaks.

FLUCOP: a toolbox for the evaluation of new flu vaccines
30/04/2019Every year, pharmaceutical companies develop vaccines designed specifically to combat the strains of influenza (flu) that are most likely to be in circulation the following winter. However, accurately predicting how much protection a new vaccine would actually offer against emerging virus types is far from easy. In the run-up to World Immunisation Week 2019, FLUCOP project coordinator Emanuele Montomoli told the IMI Programme Office how the project is developing tools to address this challenge.
ADVANCE: Towards a pan-European vaccination monitoring system
26/04/2019Vaccines are one of the most effective public health measures out, yet public distrust in immunisation programmes is limiting high vaccine uptake, resulting in outbreaks of vaccine-preventable infectious diseases that had almost disappeared. In the run-up to World Immunisation Week 2019, ADVANCE project coordinator Miriam Sturkenboom told the IMI Programme Office about the project's efforts to create an open system for actively monitoring vaccine coverage, benefits and risks in Europe.

ZAPI: Finding new ways to fight new zoonoses
25/04/2019Zoonoses are infectious diseases that can be transmitted to humans from animals (and vice versa). IMI's ZAPI project is working to create new platforms and technologies that will facilitate a fast, coordinated, and practical response to new infectious diseases as soon as they emerge. In the run-up to World Immunisation Week 2019, the IMI Programme Office caught up with ZAPI project coordinator Jean-Christophe Audonnet for an update on the project's progress so far.

How can stem cells enhance the drug discovery process? An interview with the StemBANCC coordinators
10/04/2019Scientists in the StemBANCC project successfully developed disease-relevant human induced pluripotent stem cells (iPSCs) for use as a trustworthy tool for the screening and toxicology testing of new drugs, as well as for in vitro disease modelling. In an interview with the IMI Programme Office, project coordinators Martin Graf of F. Hoffmann La Roche, and Zameel Cader of Oxford University explained the benefits of drug development through a stem cell platform.
Support for patients with respiratory diseases
13/03/2019IMI's PRO-active project has developed new patient-centred tools and approaches to help people with chronic obstructive respiratory disease (COPD) get more personalised treatments - a means to boost their activity levels, health and well-being.

A novel, preventive approach to treating rheumatoid arthritis
12/02/2019Novel therapies being developed by IMI researchers could prevent and cure rheumatoid arthritis by tackling the autoimmune disease in the very early stages, before debilitating symptoms occur.
‘We brought target binding kinetics to the international research community’ – an interview with the K4DD project coordinators
11/02/2019There is mounting evidence to suggest that the kinetics of the interaction between a drug and its target – target binding kinetics – has a strong influence on the clinical success of a drug. IMI’s K4DD project developed methods and tools to allow researchers to study drug-target interactions with greater ease. In an interview with the IMI Programme Office, project coordinator Anke Mueller-Fahrnow of Bayer and academic coordinator Ad IJzerman of Leiden University explain how the project transformed the field and could lead to more effective drugs in the future.
‘IMI really was transformative’ – an interview with the COMPACT project coordinators
28/01/2019Biological medicines, such as proteins and nucleic acids, can be much more efficient than traditional synthetic drugs. However, getting them to where they are needed in the body is a big challenge. IMI’s COMPACT project developed prototypes of new drug delivery systems, which may lead to the next generation of biopharmaceuticals. In an interview with the IMI Programme Office, project coordinator Ekkehard Leberer of Sanofi and academic coordinator Enrico Mastrobattista of the Utrecht University explain how the project transformed the biologics field, and why this wouldn’t have been possible without IMI.

IMI was a catalyst for a process that would have taken much longer otherwise – an interview with the ADAPT-SMART project coordinators
25/01/2019Today’s paradigms of bringing innovation to patients are challenged by new developments, such as a growing patient demand for timely access to promising therapies. To address this, more adaptive drug pathways are needed and the ADAPT-SMART project set out to address that challenge. In an interview with the IMI Programme office, project leader Hans-Georg Eichler of the European Medicines Agency (EMA), project coordinator Andre Broekmans of Lygature and deputy project leader Solange Rohou of AstraZeneca explained how the project created a change in the mindsets of players in the healthcare ecosystem, and how this will affect drug development in the future.

TRISTAN project reveals impact of drug side effects in lungs
11/12/2018Drugs used to treat a wide range of conditions may carry a higher risk of side effects for the lungs than previously thought. This is the result of a review by IMI’s TRISTAN project published in the Journal of Clinical Medicine. The team notes that while the drugs studied work well for most patients, doctors should be more aware of the potential risks to their patients’ respiratory systems.

Taking a tailored approach to type 2 diabetes
16/11/2018IMI's RHAPSODY project researchers have split type 2 diabetes into subgroups and are working on a tool to personalise treatment for the disease. Their aim is to help patients get the best possible care for their specific condition and cut public health costs.

DDMoRe project outputs are making drug development more transparent and credible – an interview with the project coordinators
15/10/2018In drug development, computer-based models are vital for understanding drug-related benefits and risks, but until now it has been difficult for researchers to pool their knowledge and develop these models collaboratively. To facilitate the sharing of models across sectors and organisations, the DDMoRe project developed common standards and the largest repository for clinical trial data models to date. In an interview with the IMI Programme Office, project coordinator Lutz Harnisch of Pfizer and academic coordinator Mats Karlsson of Uppsala University explain how the project outputs are already speeding up the drug development process while at the same time making it more collaborative and transparent.

Fast, effective treatment for autoimmune rheumatic diseases
12/10/2018Autoimmune rheumatic diseases like rheumatoid arthritis, lupus and scleroderma are debilitating and occasionally life-threatening. IMI's PRECISESADS project aims to improve treatment by getting the right therapies to the right patients, fast.

Remote monitoring boost for long-term patients
11/10/2018Patients with long-term medical conditions, such as depression, multiple sclerosis and epilepsy, need regular monitoring, but having to visit the clinic frequently for tests can be a problem. IMI's RADAR-CNS project is aiming to implement real-time, on-demand monitoring through remote assessment, to potentially improve patient experience and outcomes and increase clinical efficiency.

What once took months, now takes seconds – an interview with the Open PHACTS project coordinators
02/10/2018Open PHACTS has delivered an online platform that links up diverse and complementary drug discovery databases, allowing researchers to rapidly find and access relevant data. In an interview with the IMI Programme Office, academic coordinator Gerhard Ecker of the University of Vienna and EFPIA representative Derek Marren of Eli Lilly, explain how the platform is already saving researchers significant amounts of time and money, thus speeding up the drug discovery process.
‘This has been a critical investment for European researchers’ – an interview with the PreDiCT-TB project coordinators
26/09/2018Tuberculosis (TB) is still the most deadly infectious disease worldwide, yet there have been few new TB drugs approved since the 1970s. This is because the disease is extremely complex, and the laboratory tools scientists have to predict the success of potential treatments in the clinic have historically been unreliable. IMI’s PreDiCT-TB project developed new tools and biomarkers, which could help drug companies make better-informed decisions on which drugs to pursue in clinical trials. In an interview with the IMI Programme Office, EFPIA project coordinator Justin Green of GSK and academic coordinator Gerry Davies of the University of Liverpool, explain how PreDiCT-TB transformed the tuberculosis field and could speed up the development of new treatments for the benefit of patients.

‘The public-private partnership allowed us to do this in a very rapid time frame’ – an interview with WEB-RADR project coordinators
24/09/2018WEB-RADR developed a mobile app that allows users to report adverse drug reactions and receive reliable information about their treatments. The project also developed methods to extract and analyse information from social media posts about drug use and misuse. In an interview with the IMI Programme Office, project coordinators, David Lewis of Novartis and Phil Tregunno of the UK’s Medicines and Healthcare products Regulatory Agency, explain how the project outputs are already helping in the earlier detection of drug safety issues for the benefit of patients.

Boosting the fight against drug-resistant bacteria in hospitals
03/09/2018IMI's COMBACTE-MAGNET project has built an extensive public-private network to speed up the development of treatments to combat antibiotic-resistant bacteria that cause infections in intensive care units, helping address a major issue for hospitals.
‘We found a way to share the unsharable’ – an interview with the eTOX project coordinators
31/07/2018The eTOX project partners developed innovative strategies and novel software tools to better predict the safety and side effects of new candidate medicines for patients. In an interview with the IMI Programme Office, project coordinator Francois Pognan of Novartis, and academic coordinator Ferran Sanz of Institut Hospital del Mar d'Investigacions Mèdiques, explain how the tools developed by the project are already helping pharmaceutical companies make better-informed decisions in their pursuit of developing safer drugs for patients.

‘Radical collaboration’ is shaking up the pharmaceutical industry – Carlos Moedas
28/06/2018‘Radical collaboration’ where multinational companies work together and share data instead of keeping it secret is helping to change the model of the pharmaceutical industry and solve problems more quickly, according to Carlos Moedas, the EU’s commissioner for research, science and innovation.

Mobile technology solutions to monitoring medicines
21/06/2018IMI's WEB-RADR project has developed a mobile app enabling patients and healthcare professionals to more easily report suspected adverse drug reactions. It has also analysed social media to assess drug safety. Both initiatives have shown potential for strengthening the monitoring of medicines.
Developing liquid biopsy tests to diagnose cancer
14/06/2018Liquid biopsies can help to diagnose cancer in its early stages, and assist clinicians in monitoring the impact of treatment at any point. IMI's CANCER-ID project has validated a series of tests for cancer cells to help develop more effective methods of diagnosis and more personalised treatments for lung and breast cancer - two of the most frequent malignancies in Europe.
A faster way to spot the tell-tale signs of cancer-causing drugs
08/06/2018IMI's MARCAR project has identified potential biomarkers that could indicate how likely a drug is to cause cancer at an earlier stage of testing than is currently possible, which could save drug companies time and money – and lead to safer drugs reaching patients faster.

Complex diseases get the big data treatment
23/05/2018The big data explosion, which allows scientists to analyse factors such as people’s lifestyles, genes and medical records to develop personalised treatments for conditions, has so far mostly benefitted rare diseases with simple causes. But now, thanks to IMI's BigData @ Heart and ROADMAP projects, complex problems such as cardiovascular disease and dementia are getting the big data treatment.
‘The benefits of collaboration come through loud and clear’ – an interview with the GETREAL project coordinator
22/05/2018GETREAL developed new tools and resources for incorporating real-life data into drug development, which could increase confidence in new medicines and help to get them to patients more quickly. In an interview with the IMI Programme Office, project coordinator, Elaine Irving of GlaxoSmithKline explains the main project achievements, and why they wouldn’t have been possible without IMI.

Rise in vaccine hesitancy related to pursuit of purity – Prof. Heidi Larson
26/04/2018The rise of alternative health practices and a quest for purity can partly explain the falling confidence in vaccines which is driving outbreaks of preventable diseases such as measles, according to Heidi Larson, professor of anthropology, risk and decision medicine at the UK’s London School of Hygiene & Tropical Medicine, who is involved in two IMI projects: EBODAC and ADVANCE. In her interview with the Horizon Magazine, she explains how the two IMI projects are contributing to understanding drivers of vaccine confidence and developing interventions to build trust.

Tapping the full potential of Europe's health data
17/04/2018IMI's EMIF project is helping researchers tap into Europe's treasure trove of electronic health data, saving them time and money in their quest to cure and develop better drugs for debilitating diseases. The effort has already led to promising findings linked to Alzheimer's and obesity.

‘We made significant breakthroughs’ – an interview with the PREDECT project coordinators
16/04/2018IMI’s PREDECT project developed new, much-improved laboratory models of cancer, which could improve the accuracy with which pharmaceutical companies predict the effectiveness of new drugs. In an interview the IMI Programme Office, project coordinator John Hickman of Servier, and academic coordinator Emmy Verschuren of the Institute for Molecular Medicine Finland at the University of Helsinki, explain the project’s most significant breakthroughs, and how these achievements will help in the future search for new drugs.

‘We made significant accomplishments in a very challenging field’ – an interview with MIP-DILI project coordinators
09/04/2018IMI’s MIP-DILI project deepened the knowledge of the science behind drug-induced liver injury (DILI) and improved laboratory tests in use to predict DILI in the early stages of drug development. In an interview with the IMI Programme Office, Richard Weaver of Servier and academic coordinator, Kevin Park of the University of Liverpool, describe the project’s most important achievements, and explain how they are already benefitting industry, academia, and patients, while at the same time reducing the use of animals in research.

Patients are already benefiting from our project – an interview with the OncoTrack coordinators
05/04/2018IMI’s OncoTrack project developed colon cancer models which allow researchers to more accurately predict the effect of drugs on different types of colon cancer tumours. This and other project outputs are already helping some doctors in choosing the right drug for the right patient, and could lead to more effective treatments in the future. In an interview with the IMI Programme Office, project coordinator, David Henderson of Bayer, and academic coordinator Hans Lehrach of the Max Planck Institute for Molecular Genomics, explain why all this wouldn’t have been possible without the public-private collaboration brought by IMI.

‘EUPATI has been a game-changer in the empowerment of patients’ – an interview with the project coordinators
20/03/2018EUPATI built a much-needed programme for patient involvement in medicines research and development, and has been a game changer for patient empowerment in Europe, building a movement that will long outlive the project itself. In an interview with the IMI Programme Office, project coordinator Jan Geissler of the European Patients Forum, and Matthias Gottwald of Bayer explain how patients in Europe and beyond are benefitting from the work that was done.

Faster access to better and safer medicines
05/03/2018A potential drawback of clinical trials of new drugs is that they do not always reflect how well these will work in the 'real world'. IMI's GetReal project brought together key stakeholders to help add more such evidence into the design of trials, increasing confidence in new medicines and helping to get them to patients more quickly.

European Lead Factory is leading the way to discovering new medicines
26/02/2018IMI's European Lead Factory project has created an integrated platform which is providing an innovative range of free services, expertise and a huge collection of compounds for researchers who are developing new drugs to treat all types of human diseases.

SafeSciMET project trained more than 800 people in drug safety - an interview with project coordinators
06/02/2018IMI’s SafeSciMET project established a much-needed education and training programme in drug safety sciences in Europe. In an interview with the IMI Programme Office, project coordinators Manfred Kansy of Roche and Nico Vermeulen of the Vrije Universiteit Amsterdam explain how this project will contribute to developing safer medicines in the future.

'All this wouldn’t have been possible without IMI' - an interview with Eu2P project coordinators
01/02/2018Eu2P developed the first internationally recognised European online education & training programmes in pharmacovigilance and pharmacoepidemiology. In an interview with the IMI Programme Office, academic project coordinator Annie Fourrier-Réglat and project manager Karine Palin, both of the University of Bordeaux, reveal the long-lasting benefits that this project will bring to academia and industry.

‘It was a once-in-a-lifetime chance to make this happen’ – an interview with the PharmaTrain project coordinators
30/01/2018IMI’s PharmaTrain project provided much needed harmonisation and coordination among European universities offering medicines development education and training. In an interview with the IMI Programme Office, former project coordinator Ingrid Klingmann, now president of the successor organisation, PharmaTrain Federation asbl, and Matthias Gottwald of Bayer, explain why this was an important opportunity, and why universities around the world are envious that Europe could make such a project happen.

Thanks to EMTRAIN, we got out of our ivory towers – an interview with project coordinators
25/01/2018EMTRAIN established a pan-European platform for education and training covering the whole life cycle of medicines research, from basic science through clinical development to pharmacovigilance. In an interview with the IMI Programme Office, industry co-coordinator Matthias Gottwald of Bayer and academic coordinator Michael Wolzt of the Medical University of Vienna, explain what the project achieved and how it benefitted both industry and the academic community.

We are more prepared for future Ebola outbreaks – an interview with the Mofina project coordinator
23/01/2018IMI’s Mofina project developed a portable device which can test for deadly Ebola in 75 minutes or less, eliminating the need to take suspected Ebola patients to treatment centres far away of their communities. In an interview with the IMI Programme office, the project’s coordinator, Edmund Newman of Public Health England, explains how Mofina’s success will save lives, and help contain future outbreaks.

Green manufacturing for the pharmaceutical industry
11/12/2017IMI's CHEM21 project has developed environmentally friendly chemistry processes for drug manufacturing. As well as being better for the planet, the new processes will also enable the industry to cut costs and could lead to cheaper medicines for patients.

Stimulating the development of new antibiotics
08/12/2017Many advances of modern medicine rely heavily on antibiotics - which can, however, lose their effectiveness over time as bacteria adapt. New types of these precious drugs are urgently needed. IMI's DRIVE-AB researchers are looking into ways to foster the required innovation.
Developing a fast, local test for deadly Ebola
01/12/2017Rapid diagnosis is vital for controlling outbreaks of the deadly Ebola virus. Currently this can only be done in complex laboratories and samples from infected patients are dangerous to handle and transport. Faced with this challenge, IMI's EbolaMoDRAD project is developing fast, local tests to spot infection quickly and safely, helping to contain its spread and saving lives in the process.
IMI projects delivering quality and quantity on publications
29/11/2017The sheer volume and high quality of publications coming out of IMI projects is highlighted in the latest analysis of IMI publications carried out by Clarivate Analytics (formerly Thomson Reuters). IMI has been monitoring and analysing the papers coming out of its projects since 2012, and the number of publications is increasing year-on–year. In 2016 alone, IMI projects produced 796 publications, bringing the total number of publications produced by IMI...

First batches of ULTRA-DD patient-based assay data released - ahead of publication
27/11/2017The ULTRA-DD project has made good on its promise to make its data open source with the publication online of datasets from experiments on autoimmune diseases such as lupus and myositis. Through the experiments, the ULTRA-DD team has identified potential new targets that could inspire the development of new treatments for these diseases. The project hopes that if other researchers probe and use the data, they may uncover further insights...

New tumour models could lead to more effective treatments
27/11/2017The incidence of cancer in Europe is increasing but many potential new drug treatments are found to be ineffective when tested on patients. IMI's PREDECT project has investigated new models of tumours to help researchers discover more effective treatments and boost survival rates.
Using health records to help clinical research
31/10/2017New treatments must be tested in clinical trials to ensure they are safe and effective. IMI's EHR4CR project has enabled scientists to find suitable patients by searching millions of medical records while keeping personal data secure.

New brochure: Carrying the torch for medical innovation
26/10/2017IMI projects are accelerating the medicines development process, generating new scientific insights, and developing resources for open use by the research community. Furthermore, some of our projects are already delivering direct benefits to patients. Interested in concrete numbers and examples? Find out more about the impact and outputs of our projects in our new brochure, and learn how we are 'Carrying the torch for medical innovation'.
Biomarker tests to speed up cancer drug development
20/10/2017IMI's QuIC-ConCePT project research into validating more imaging biomarkers for use in cancer drug trials seeks to speed up development of successful new drugs and avoid exposing patients to treatment that does not work for them.
Better understanding of colon cancer to help guide treatment
28/09/2017IMI's OncoTrack project researchers have worked to identify and characterise signs of cancer, particularly colon cancers, and patients' responses to different types of treatment. The aim is to help doctors choose the best possible treatment for an individual patient's condition, potentially improving and saving lives.

EBiSC project's stem cell biobank could lead to new drugs
19/09/2017Europe has identified the need for a central, standardised stem cell repository providing researchers with access to quality controlled cell lines and data for future drug development. EU and industry funding helped this new biobank facility establish initial operations and create a 'foundational collection'.

Mofina project develops device for faster Ebola testing
18/09/2017IMI's Mofina project researchers have developed a portable device to test in the field whether a person has caught the deadly Ebola disease. It gives reliable results in 75 minutes, which can help contain outbreaks and save lives.

Pharma-Cog results may speed up Alzheimer’s drug development
13/09/2017IMI’s Pharma-Cog project had an ambitious goal: to improve the success rate of Alzheimer’s disease drugs in development. As the project draws to a close, we talked to scientific coordinator Régis Bordet of the University of Lille and EFPIA project coordinator Jill Richardson of GSK. According to them, Pharma-Cog made several important contributions to this field of research, including a better understanding of the disease, and may lead to more efficient and more precise clinical trials for future drug candidates.

RAPP-ID outputs could boost development of rapid diagnostic tests
06/09/2017The focus of RAPP-ID was the development of rapid diagnostic platforms for infectious diseases, and some of the technologies and resources developed within the project could help speed up the development of rapid diagnostic tests in the future. In an interview with the IMI Programme Office, project coordinator Jorge Villacian of Johnson & Johnson and scientific coordinator Herman Goossens of University of Antwerp, outline the challenges encountered and successes achieved.

BTCure project insights connect bone cells to rheumatoid arthritis
31/08/2017IMI's BTCure project has generated new insights into the causes and development of rheumatoid arthritis, directing efforts towards earlier detection, prevention and the idea of inducing tolerance to this chronic and debilitating disease. Follow-up research includes new studies to further explore this 'tolerance' challenge and progress on a new antibody detecting device.

ENABLE scientists discover a new way to target drug-resistant bacteria
24/06/2017Scientists from IMI’s ENABLE project have found a new mechanism to target drug-resistant bacteria, opening up a promising new pathway for further research. The mechanism involves DNA gyrase, a well-known enzyme that is a target of already existing antibiotics. ENABLE scientists found a new way to inhibit this enzyme and kill drug-resistant bacteria in the laboratory.
EPAD – revolutionising clinical trials for dementia
06/06/2017IMI’s EPAD project has an ambitious goal – to revolutionise the way we carry out clinical trials for treatments designed to prevent Alzheimer’s dementia. In an interview with the IMI Programme Office, project coordinators Craig Ritchie of the University of Edinburgh and Serge Van der Geyten of Janssen Pharmaceutica explain what the project has achieved so far and why the project team is like a family.
VSV-EBOVAC identifies signature of promising Ebola vaccine
10/05/2017How exactly does our immune system respond to vaccination? In the first study of its kind, scientists from IMI’s VSV-EBOVAC project, studying a promising Ebola vaccine, set out to find out which immune cells get activated early on, which inflammatory markers are released after that, and how this early activity later impacts the production of antibodies against the Ebola virus. In the process, they discovered a unique signature of a promising Ebola vaccine candidate which could not only help predict adverse reactions and effectiveness of this vaccine, but also inform the development of vaccines for other diseases as well.
Of vaccines, rumours and the success of IMI’s EBODAC project
09/03/2017When IMI’s EBODAC project started in late 2014, Ebola had already killed over 8 000 people in just a few short months, most of them in the western African nations of Liberia, Guinea and Sierra Leone. With the outbreak continuing to devastate lives across the region, fear and anxiety were rife, and rumours spread rapidly through local communities. It was against this backdrop that IMI’s EBODAC project set out to develop a community engagement strategy to enable a clinical trial of a promising new Ebola vaccine candidate. Thanks to the many innovative methods employed, including radio and drama shows, today the project celebrates its first big success: all of the adults in the trial have been successfully vaccinated. But getting there was far from easy.

'More successful than what we thought possible' – an interview with the Europain project coordinator
06/03/2017In 2009, IMI’s Europain project set out on an ambitious path: to improve the treatment of patients with chronic pain. At the end of the project, we interviewed project coordinator Märta Segerdahl of H. Lundbeck A/S, and asked her what the project had managed to achieve. ‘We ended up more successful than we thought possible,’ she said.

CHEM21 method could dramatically cut production costs of essential anti-fungal medicine
01/02/2017Scientists from the Innovative Medicines Initiative (IMI) project CHEM21 have developed a new, more efficient way of producing flucytosine, a medicine used to treat a common and often deadly fungal form of meningitis in people with HIV / AIDS. The new method, which is described in a paper in Organic Process Research & Development (OPR&D), is expected to decrease drastically costs of production, and so make the medicine more affordable for the many people with HIV / AIDS who live in low income countries. The CHEM21 team is now working to scale up the method to the industrial scale.

'It was a really fantastic experience' – an interview with the MARCAR project coordinator
13/01/2017Cancer risk assessment is one of the most important steps during the development of new medicines. Launched in 2010, IMI’s MARCAR project aimed to develop early biological clues – biomarkers – which could help predict which drugs might lead to tumour growth. At the end of the project, MARCAR project coordinator, Jonathan Moggs of Novartis, spoke to the IMI programme office about the main project achievements and explained how MARCAR project outputs will make drugs safer in the future.

'We took the lead over US-based projects' – an interview with SUMMIT project coordinators
12/01/2017Before SUMMIT started in 2009, ongoing research on diabetic complications in Europe was scattered amongst various countries. Fast forward six years, and Europe – by joining forces and activities - has climbed to the top of the research ladder in this field, coming close to or even surpassing similar research projects in the US. In an interview with the IMI project office, SUMMIT project coordinator Michael Mark of Boehringer-Ingelheim, and scientific coordinator Leif Groop of Lund University, explain how SUMMIT contributed to the diabetes complications field.

'There will be lots of benefits to patients' – an interview with the SAFE-T project coordinators
11/01/2017SAFE-T was among the first IMI projects which started in 2009. Seven years later, the project came to a close, having achieved most of its objectives and made significant progress in developing improved tools for the prediction, detection, and monitoring of drug-induced injuries to the kidney, liver, and vascular system. In an interview with the IMI Project Office, project coordinator Michael Merz of Novartis, and scientific coordinator Thomas Joos of the Natural and Medical Institute at the University of Tübingen (NMI), share their thoughts on the project’s successes.
PROactive draws to a close, delivers on its promises – an interview with project coordinators
15/11/2016PROactive was launched in 2009 with the aim of developing an innovative tool to measure physical activity in chronic obstructive pulmonary disease (COPD) patients. The project has now drawn to a close, meeting its main goal. In interview with the IMI Programme Office, PROactive’s scientific coordinator, Thierry Troosters of the University of Leuven, and project coordinator, Mario Scuri of CHIESI Pharmaceuticals, explain how the new tool works and how it will benefit patients.

'Without IMI this would have never happened' – an interview with U-BIOPRED’s Peter Sterk
11/08/2016U-BIOPRED was one of the first IMI projects to launch back in 2009. Now the project, which focused on severe asthma, is drawing to a close. In an interview with the IMI Programme Office, the project’s scientific coordinator, Peter Sterk of the University of Amsterdam, explains how the project has increased our understanding of severe asthma and how researchers are already using this knowledge in the development of new treatments. He also talks about how the project benefited from the involvement of patients, and how they put the concept of ‘big data’ into practice to achieve their groundbreaking results.

