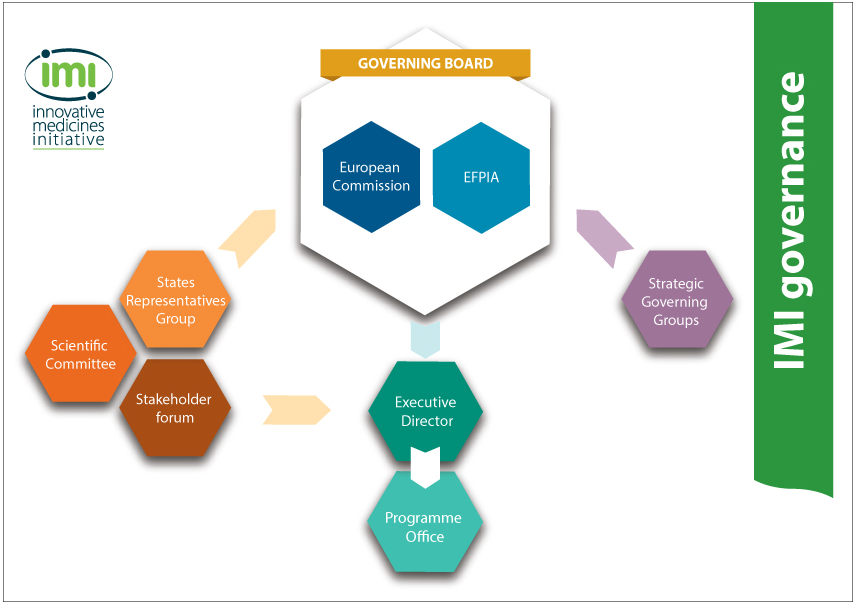
Governance
The Innovative Medicines Initiative (IMI) is a public-private partnership (PPP) between the European Union, represented by the European Commission, and the European Federation for Pharmaceutical Industries and Associations (EFPIA).
Our governance structure includes the following:
-
The IMI Governing Board is our highest decision-making body. It is composed of an equal number of representatives from our two founding members, namely the European Commission and EFPIA.
-
The IMI Scientific Committee is made up of scientific experts from diverse fields and provides us with high-level recommendations.
-
The IMI States Representatives Group consists of representatives of the EU Member States and the countries associated to the EU's research programmes. It provides opinions on scientific, administrative and financial matters.
-
The Strategic Governing Groups comprise representatives of pharmaceutical companies as well as people from the European Commission and the IMI Scientific Committee. They develop our Call topics, and ensure coordination of our projects in key areas with each other and with the European Commission's wider research programmes.
-
The IMI Executive Director is responsible for our day-to-day management and heads up our Programme Office.
-
Held annually, the IMI Stakeholder Forum brings together our stakeholders to learn about our latest activities and plans, and provide us with their feedback.
More broadly, organisations such as the European Court of Auditors and the European Parliament also play an important role in our governance through their oversight of our budgetary control procedures.
