10 years of transforming medical research

30 April 2018 marked 10 years since our first Call for proposals. Since then, we’ve been carrying the torch of medical innovation, helping to deliver breakthroughs that – step-by-step – are bringing about a healthier future for us all. We are doing this by finding new ways for experts, industry and academia to share insights, data and knowledge, making it possible for the best minds to collaborate to speed up vital research and work to overcome some of the biggest medical challenges facing humanity.

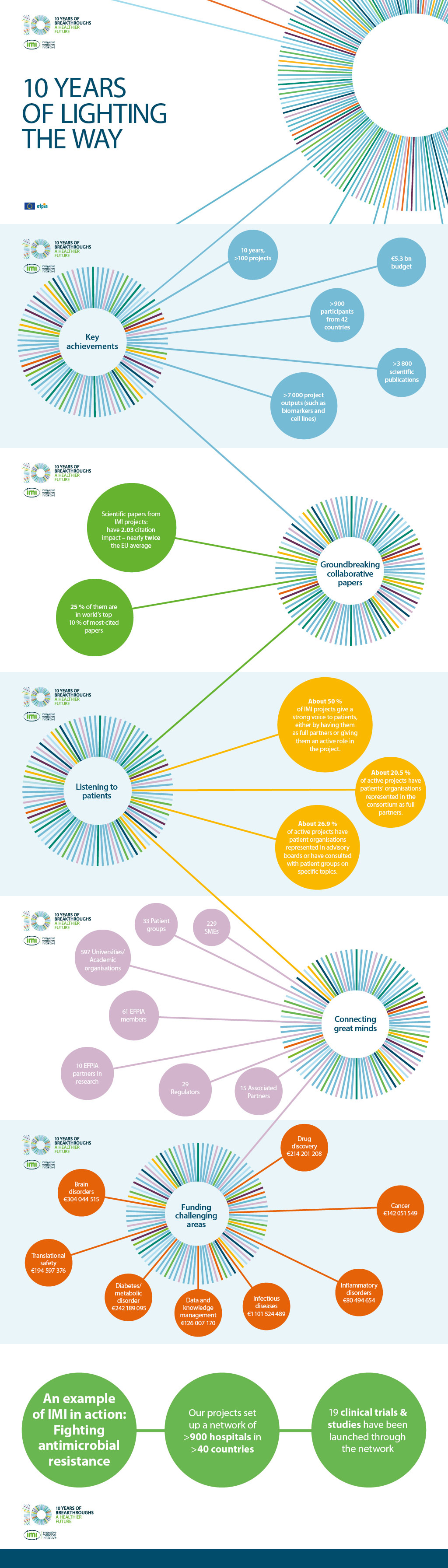
Our projects – almost 100 and counting – all take a collaborative, open innovation approach to some of the biggest challenges in medical research and drug development today, such as severe asthma, Alzheimer’s disease, diabetes and cancer. By providing a unique model of collaboration, they are laying the foundations that are helping researchers across Europe, including many in SMEs, to advance their drug development programmes. These new, open partnerships enable the IMI-funded consortia to cover the entire value chain and contribute to more rapidly bringing new products or treatments to market for the benefit of patients.
We’ve achieved so much in the past ten years and we thought that was worth celebrating.
Therefore, throughout 2018 we had a number of activities taking place to mark the occasion, including the events ‘IMI: Celebrating 10 Years of Medical Innovations’, the Scientific Symposium and the IMI Stakeholder Forum.
To mark our anniversary, we also created a series of video testimonials from our key partners and stakeholders.
The Story of the Innovative Medicines Initiative: Rudolf Strohmeier, Director-General of the Publications Office of the European Union and Former Chair of the IMI Governing Board; Nathalie Moll, Director-General, EFPIA
IMI 10th Anniversary General Video:
Watch more IMI's 10th anniversary on YouTube.
You can also catch up on IMI's 10th anniversary on Twitter, via our hashtags #IMI10years and #IMICarryTheTorch, and LinkedIn.
Want to learn more about the IMI story? Read about IMI’s history and learn how the EU and the pharmaceutical industry teamed up to create IMI.
IMI is funded jointly by the European Union (represented by the European Commission) and the European pharmaceutical industry (represented by EFPIA, the European Federation of Pharmaceutical Industries and Associations). The EU funding comes from Horizon 2020 and the EU's Seventh Framework Programme for Research (FP7).