The European Commission has officially granted market authorisation for an IMI-supported Ebola vaccine regimen, which represents a vital tool in the fight against the deadly disease.
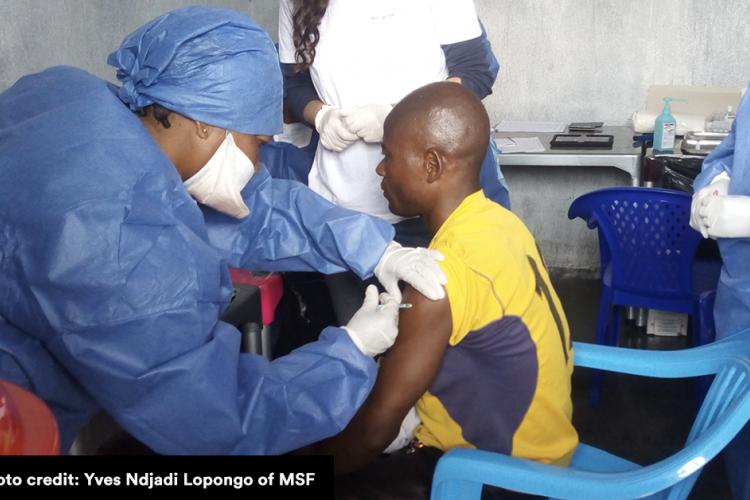
Today’s announcement grants marketing authorisation to Janssen, a Johnson & Johnson company, for its two-dose Ebola vaccine regimen. The ‘prime-boost’ regimen comprises two components - Zabdeno® (Ad26.ZEBOV) and Mvabea® (MVA-BN-Filo).
It is specifically designed to induce long-term immunity against the Ebola virus in adults and children aged one year and up. As such, it can be used to support preventive vaccination in countries most at risk of outbreaks.
A number of organisations contributed to the development of the vaccine regimen, including IMI through the Ebola+ programme.
IMI launched the Ebola+ programme in 2014 in rapid response to the Ebola outbreak in western Africa. Through the EBOVAC projects, IMI has supported several clinical trials of the J&J vaccine regimen in Africa and Europe. In addition, IMI’s EBODAC project successfully developed tools to engage local communities in the trials, while the EBOMAN project supported the large-scale manufacture of the vaccine regimen.
‘IMI is immensely proud to have contributed to the development of this much-needed Ebola vaccine regimen. Through our Ebola+ projects, we brought together some of the world’s leading experts in Ebola from universities, the pharmaceutical industry, small companies, and the charity sector in Europe, Africa and the US,’ said IMI Executive Director Pierre Meulien. ‘By working together, often in challenging circumstances, they were able to significantly advance the development of this Ebola vaccine regimen and so pave the way for today’s decision.’
‘Today, we can be glad to have supported the development of the Ebola vaccine with EU funding, in partnership with the European pharmaceutical sector under the Innovative Medicines Initiative,’ said Mariya Gabriel, European Commissioner in charge of Research. ‘The investment from the EU's research programme Horizon 2020 into several Innovative Medicines Initiative Ebola projects is now bearing fruit. This demonstrates, yet again, the power of collaboration and European R&I leadership to tackle global health threats.’
Ebola virus disease spreads through direct contact with the bodily fluids of infected patients who are showing symptoms. It usually begins with flu-like symptoms, but rapidly progresses to multiple organ failure and blood-clotting abnormalities which manifest as internal and external haemorrhages (bleeding). It is fatal in between 25% and 90% of cases.
The outbreak in western Africa in 2014-2016 infected over 28 000 people, mainly in Guinea, Liberia, and Sierra Leone, and killed 11 000. More recently, an outbreak in the east of the Democratic Republic of the Congo (DRC) infected almost 3 500 and killed over 2 000. The WHO recently declared the eastern DRC outbreak over, but now a new (unrelated) cluster of cases has emerged in the west of the country. These outbreaks have shown how quickly the disease can spread, and underline the need for safe, effective vaccines as well as treatments and diagnostic tests.
Read more
European Commission’s press release
Latest COVID-19 vaccine based on technology tested for safety & immunogenicity in IMI Ebola projects
Johnson & Johnson’s press release
More success stories from IMI's Ebola+ programme
A recipe for the next disaster: a new, pan-virus methodology for ramping up vaccine production
